Evaluation process held every 5 years
Legal basis
When the ERNs were established, it was agreed that an evaluation would be conducted every five years after their initial approval (or last evaluation) to determine the worth or significance of the work and actions developed by the ERNs. In 2022/2023, the ERNs and our member HCPs from the 1st call underwent a formal performance evaluation for the period 2017-2021. The report of this evaluation has been published in December 2024 (link to the report).
The first evaluation of the ERNs was initiated by the European Commission in December 2022, five years after their inception. This evaluation was designed to verify and assess:
- The fulfilment of the criteria and conditions in Delegated Decision 2014/286/EU,
- Achievement of the objectives set out in Article 12(2) of Directive 2011/24/EU,
- The outcomes and performance of the networks, including the contributions of individual member healthcare providers (HCPs).
Evaluation process
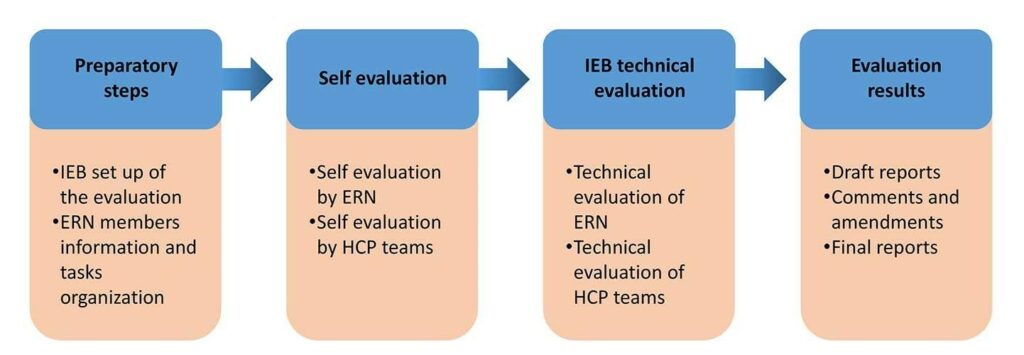
The evaluation process consisted of preparatory tasks and three main steps, ensuring a thorough and structured assessment of the European Reference Networks (ERNs).
Timeline of the evaluation process: IEB ; ERN (European Reference Network for Rare Diseases); Clinical Centres (CC)
Origin of the figure ERNs Evaluation – European Commission
Preparatory Tasks
The preparatory phase involved organizational, managerial, logistical, and communication arrangements, all of which were carried out by the Independent Evaluation Body (IEB).
Main Steps
- Self-Evaluation
Each ERN and its members conducted a self-assessment to determine whether the ERNs were meeting their original objectives. - Technical Evaluation
This phase included: Interviews with key stakeholders, documentation review and on-site or online audits conducted by the IEB. - Report Drafting
Reports were prepared for: each individual ERN, each ERN member and the ERN system as a whole.
Stakeholder Engagement
Throughout the evaluation process, input was sought from all relevant stakeholders, including: ERN members, the Health Care Providers, the Board of Member States, ERN coordinators and Patient organizations.
Evaluation results of the first five years evaluation
In 2022 and 2023, the 24 ERNs, along with member HCPs from the first call, underwent their first evaluation, covering the period from 2017 to 2021. The results of this evaluation were compiled into a report published in December 2024 (accessible under “Useful Links”).
Extract from the ERNs Evaluation report – European Commission
In 2023, 24 European Reference Networks, including 836 members, completed their first evaluation. Overall, the evaluation concluded that the ERN ecosystem is functioning well, meaning they are delivering on highly specialist work for rare disease patients such as consultations for diagnosis and therapies, the production of clinical guidelines and specialised trainings. 100% of the ERN networks and 88% of their members obtained satisfactory results, while only 4 % of the members saw their membership terminated. 72 Clinical Centres had to implement “Improvement Plans” and will go through the re-evaluation process in 2025.
For ERN-EYE, the evaluation of its first five years (2017-2021) was completed with success.
Monitoring data collection from ERN-EYE Health care providers
Annual Monitoring data collection
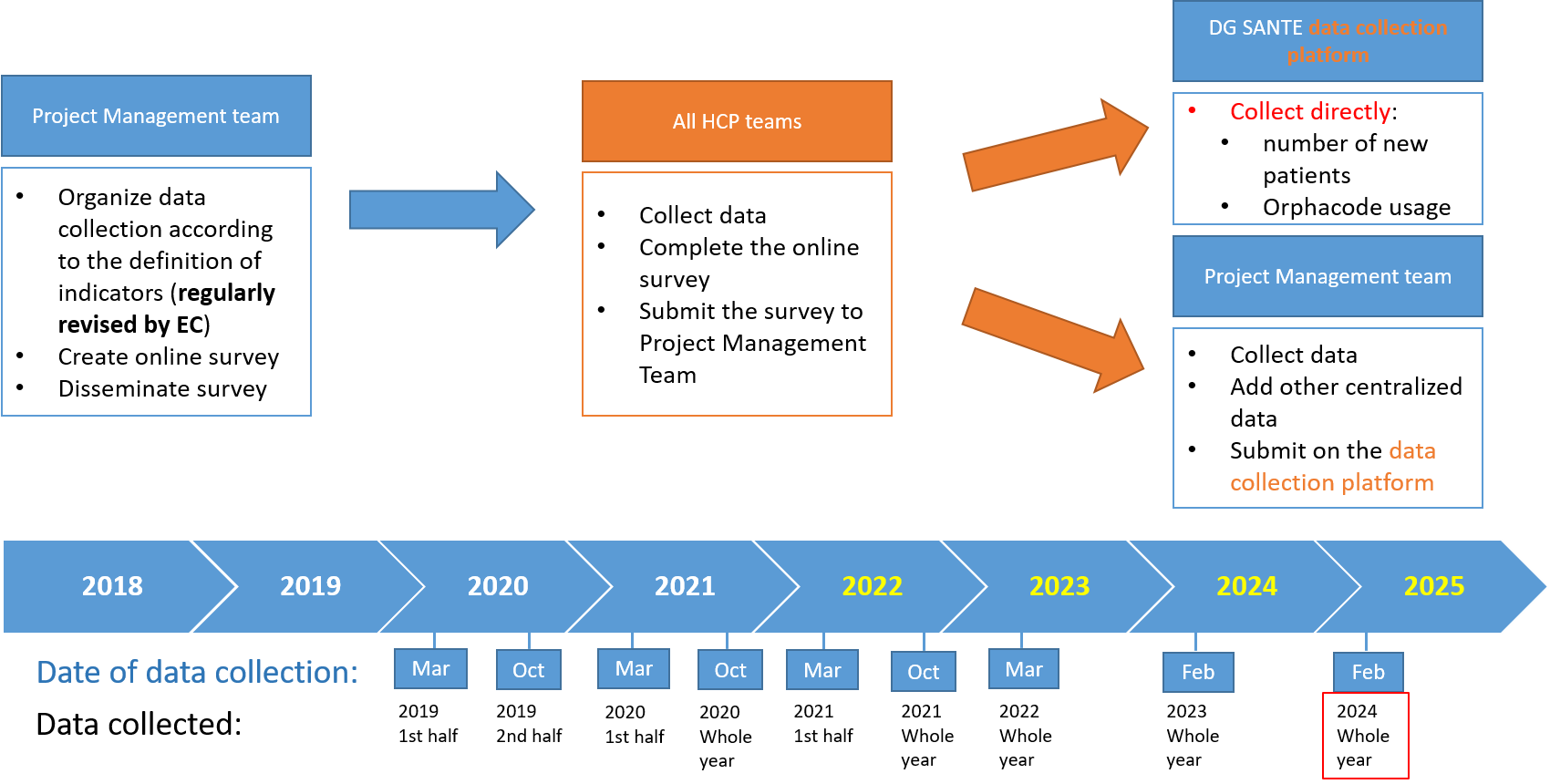
From 2023, the data collection is organised on annual basis. This initial data collection was based on 18 indicators defined by the European commission and the ERN monitoring Working Group. Since 2024, the indicators were enriched to 24 indicators (list available under useful links) and some indicators are directly collected from members HCPs.
Monitoring process
The indicators are collected via two processes:
- Directly by the European Commission through the data collection platform
- Through a survey launched by the ERN-EYE Coordination team
The next monitoring collection for 2025 is expected to start in January 2025.
Data Collection Process for 2025 (Reporting 2024 Data)
The 2025 data collection process, based on 2024 data, will begin on January 1, 2025, and conclude on March 31, 2025.
- Stage 1 (1 January- 28 February 2025):
Health Care Providers (HCPs) will submit their indicators to both the European Commission (EC) and the European Reference Networks (ERNs). - Stage 2 (March 2025):
The ERNs will submit the collected and analysed data to the European Commission (EC).
The ERN-EYE management team will launch the survey to collect the additional indicators beginning of January 2025.

